Daratumumab, a monoclonal antibody, has revolutionized the treatment landscape for hematologic malignancies. It targets CD38, a protein expressed on the surface of multiple myeloma and other cancer cells, leading to their destruction through various mechanisms. This targeted approach has shown promising results in clinical trials, demonstrating its potential to significantly improve patient outcomes.
The development of Daratumumab stemmed from the understanding of the role of CD38 in the pathogenesis of hematologic malignancies. Its unique mechanism of action, involving antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), has paved the way for new treatment strategies, particularly for patients with relapsed or refractory disease.
Daratumumab
Daratumumab is a groundbreaking monoclonal antibody that has revolutionized the treatment of multiple myeloma and other hematologic malignancies. It targets CD38, a protein found on the surface of myeloma cells and other immune cells, leading to their destruction.
Mechanism of Action
Daratumumab works by binding to CD38, a protein expressed on the surface of myeloma cells and other immune cells. This binding triggers several mechanisms that lead to the elimination of myeloma cells, including:
* Antibody-dependent cellular cytotoxicity (ADCC): Daratumumab binds to CD38 on myeloma cells, making them susceptible to attack by natural killer (NK) cells, which are part of the body’s immune system. NK cells recognize the antibody-bound myeloma cells and release cytotoxic molecules that kill them.
* Complement-dependent cytotoxicity (CDC): Daratumumab activates the complement system, a part of the immune system that helps destroy cells. When daratumumab binds to CD38, it triggers the complement cascade, leading to the formation of a membrane attack complex (MAC) that punches holes in the myeloma cell membrane, causing cell death.
* Direct cell death: Daratumumab can also directly induce apoptosis (programmed cell death) in myeloma cells by interfering with their signaling pathways.
Therapeutic Targets
Daratumumab has shown remarkable efficacy in treating various hematologic malignancies, including:
* Multiple myeloma: Daratumumab is approved for the treatment of multiple myeloma in various settings, including newly diagnosed, relapsed, and refractory disease. It is often used in combination with other therapies, such as chemotherapy, proteasome inhibitors, and immunomodulatory drugs.
* Acute lymphoblastic leukemia (ALL): Daratumumab has shown promising results in treating some types of ALL, particularly those expressing CD38. It is currently being investigated in clinical trials for this indication.
* Non-Hodgkin lymphoma (NHL): Some subtypes of NHL, such as Waldenström macroglobulinemia, express CD38 and may benefit from daratumumab treatment. Clinical trials are exploring its role in these malignancies.
History and Development
The development of daratumumab is a testament to the advancements in antibody engineering and immunotherapy.
* Discovery: Daratumumab was initially discovered as a monoclonal antibody that specifically targets CD38. It was developed by Genmab A/S, a Danish biotechnology company.
* Preclinical studies: Preclinical studies demonstrated the effectiveness of daratumumab in killing myeloma cells and other hematologic malignancy cells in vitro and in vivo.
* Clinical trials: Daratumumab underwent extensive clinical trials to evaluate its safety and efficacy in patients with multiple myeloma and other hematologic malignancies. These trials showed significant improvements in overall survival, progression-free survival, and response rates.
* FDA approval: Based on the positive results of clinical trials, daratumumab was approved by the U.S. Food and Drug Administration (FDA) in 2015 for the treatment of multiple myeloma.
Clinical Applications
Daratumumab has been successfully used in various clinical settings for the treatment of hematologic malignancies, including:
* First-line therapy: Daratumumab is now a standard of care in the first-line treatment of multiple myeloma, often used in combination with other therapies.
* Relapsed/refractory disease: Daratumumab is highly effective in treating patients with relapsed or refractory multiple myeloma who have not responded to previous therapies.
* Maintenance therapy: Daratumumab can be used as maintenance therapy after achieving a complete or partial response to initial treatment, helping to prolong remission and prevent disease recurrence.
Current Research
Research continues to explore the potential of daratumumab in treating other hematologic malignancies and in combination with novel therapies.
* Combination therapies: Ongoing clinical trials are evaluating the efficacy of daratumumab in combination with other promising drugs, such as CAR T-cell therapy and bispecific antibodies.
* New indications: Researchers are investigating the potential of daratumumab in treating other cancers, such as solid tumors, that express CD38.
Daratumumab has revolutionized the treatment of multiple myeloma and other hematologic malignancies, offering patients a new and effective weapon in their fight against cancer. As research continues, we can expect even more innovative applications of this groundbreaking monoclonal antibody in the future.
Clinical Applications of Daratumumab
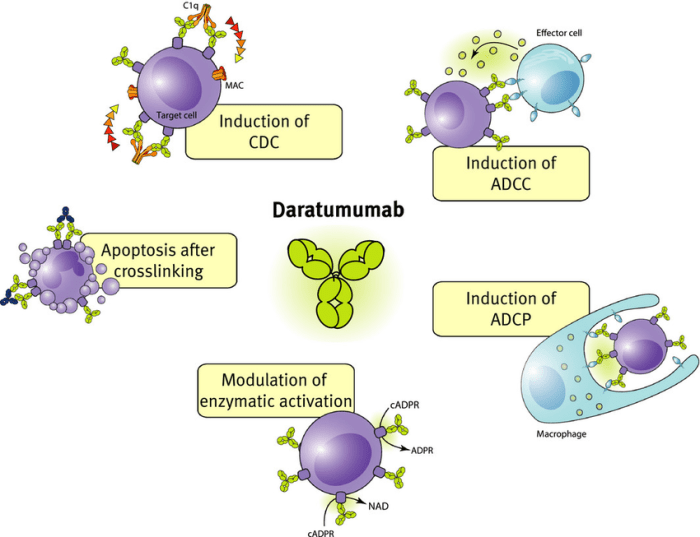
Daratumumab, a human monoclonal antibody targeting CD38, has revolutionized the treatment landscape for multiple myeloma and other hematologic malignancies. Its unique mechanism of action and impressive clinical efficacy have solidified its place as a cornerstone therapy in various treatment settings.
Approved Indications for Daratumumab
Daratumumab has received approval from regulatory agencies worldwide for several indications in hematologic malignancies. Its approved uses are summarized below:
- Multiple Myeloma: Daratumumab is approved for the treatment of patients with multiple myeloma in various settings, including:
- Newly diagnosed multiple myeloma: In combination with lenalidomide and dexamethasone (Rd) or bortezomib and dexamethasone (Vd) in patients who are eligible for autologous stem cell transplantation.
- Relapsed/refractory multiple myeloma: In combination with pomalidomide and dexamethasone (Pd) or with carfilzomib and dexamethasone (Kd) in patients who have received at least one prior therapy.
- Maintenance therapy: Following induction therapy and autologous stem cell transplantation in patients with newly diagnosed multiple myeloma.
- Waldenström Macroglobulinemia: Daratumumab is approved in combination with rituximab, bendamustine, and dexamethasone for the treatment of patients with Waldenström macroglobulinemia who have received at least one prior therapy.
Efficacy and Safety Profiles of Daratumumab
Daratumumab’s efficacy and safety profiles have been extensively studied in clinical trials across various treatment settings. The table below summarizes the key findings for multiple myeloma:
| Treatment Setting | Efficacy (Overall Response Rate) | Safety Profile (Common Adverse Events) |
|---|---|---|
| Frontline (with Rd or Vd) | 80-90% | Infusion-related reactions, neutropenia, thrombocytopenia, pneumonia, diarrhea |
| Relapsed/Refractory (with Pd or Kd) | 60-70% | Infusion-related reactions, neutropenia, thrombocytopenia, pneumonia, diarrhea, peripheral neuropathy |
| Maintenance Therapy | Improved progression-free survival | Infusion-related reactions, neutropenia, thrombocytopenia, pneumonia, diarrhea |
Role of Daratumumab in Combination Therapies
Daratumumab’s unique mechanism of action and its ability to overcome resistance to conventional therapies have made it a valuable component of combination regimens. Its use in combination with other agents, including chemotherapy, immunotherapy, and targeted therapies, has demonstrated significant clinical benefits:
- Chemotherapy: Daratumumab has been effectively combined with various chemotherapy agents, such as lenalidomide, bortezomib, and carfilzomib, leading to improved response rates and progression-free survival in multiple myeloma patients.
- Immunotherapy: Daratumumab’s synergistic effect with other immunotherapies, such as pomalidomide and ixazomib, has been observed in clinical trials, demonstrating its potential to enhance the anti-tumor immune response.
- Targeted Therapies: Daratumumab has been investigated in combination with targeted therapies, such as the proteasome inhibitor ixazomib and the immunomodulatory drug lenalidomide, suggesting its potential to enhance the efficacy of these agents.
Pharmacokinetics and Pharmacodynamics of Daratumumab
Daratumumab, a human monoclonal antibody targeting CD38, exhibits a unique pharmacokinetic and pharmacodynamic profile, influencing its efficacy in treating hematologic malignancies. This section delves into the intricate aspects of Daratumumab’s absorption, distribution, metabolism, and excretion, along with its mechanism of action and impact on tumor cells and the immune system.
Pharmacokinetic Properties of Daratumumab
Daratumumab’s pharmacokinetic properties are characterized by its slow absorption, wide distribution, and prolonged elimination half-life.
- Absorption: Daratumumab is administered intravenously, bypassing the gastrointestinal tract, leading to rapid and complete absorption.
- Distribution: Daratumumab distributes widely throughout the body, reaching various tissues and organs, including the tumor microenvironment.
- Metabolism: Daratumumab is not metabolized in the body and is eliminated as an intact protein.
- Excretion: Daratumumab is primarily eliminated through renal excretion, with a terminal elimination half-life of approximately 28 days.
Pharmacodynamic Effects of Daratumumab
Daratumumab exerts its therapeutic effects by binding to CD38, a protein expressed on the surface of multiple myeloma cells, as well as on normal cells like lymphocytes and some immune cells. This binding triggers a cascade of events that ultimately lead to tumor cell death and immune system modulation.
- Mechanism of Action: Daratumumab’s primary mechanism of action involves antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and direct induction of apoptosis in myeloma cells.
- Impact on Tumor Cells: Daratumumab’s binding to CD38 on myeloma cells activates immune effector cells, such as natural killer (NK) cells and macrophages, to kill tumor cells through ADCC. Additionally, the antibody triggers the complement cascade, leading to CDC and tumor cell lysis. Furthermore, Daratumumab can directly induce apoptosis in myeloma cells, contributing to tumor cell death.
- Impact on the Immune System: Daratumumab’s binding to CD38 on immune cells, particularly lymphocytes, can modulate immune responses. While the exact mechanisms are still under investigation, some studies suggest that Daratumumab may enhance the activity of certain immune cells, potentially contributing to its antitumor effects.
Summary of Pharmacokinetic and Pharmacodynamic Parameters of Daratumumab
| Parameter | Value |
|---|---|
| Route of Administration | Intravenous |
| Absorption | Rapid and complete |
| Distribution | Wide |
| Metabolism | Not metabolized |
| Elimination Half-Life | ~28 days |
| Mechanism of Action | ADCC, CDC, Apoptosis induction |
| Target | CD38 |
| Therapeutic Effects | Tumor cell death, Immune modulation |
Safety and Adverse Effects of Daratumumab
Daratumumab, a monoclonal antibody targeting CD38, has shown remarkable efficacy in treating various hematologic malignancies. However, like any therapeutic agent, it can be associated with adverse effects. Understanding these potential risks is crucial for informed decision-making in patient management.
Common Adverse Effects
Common adverse effects of Daratumumab are generally mild to moderate in severity. These include:
- Infusion-related reactions (IRR): These are the most frequent adverse effect, occurring in approximately 20-30% of patients. IRRs are usually mild and self-limiting, often manifested as fever, chills, and hypotension. Pre-medication with antihistamines, corticosteroids, and acetaminophen can help minimize their occurrence and severity.
- Infections: Daratumumab can suppress the immune system, increasing the risk of infections. These can range from common colds to more serious bacterial, viral, or fungal infections. Monitoring for signs of infection and prompt treatment are essential.
- Gastrointestinal disturbances: Nausea, vomiting, diarrhea, and constipation are common adverse effects, usually manageable with supportive care.
Serious Adverse Effects
While less common, some serious adverse effects can occur with Daratumumab treatment. These include:
- Hepatotoxicity: Liver function abnormalities can occur, especially in patients with pre-existing liver disease. Regular monitoring of liver function tests is crucial.
- Hemolytic anemia: Daratumumab can cause hemolysis, a breakdown of red blood cells. This is more likely in patients with pre-existing anemia or those receiving other medications that can cause hemolysis.
- Immune-mediated adverse effects: Daratumumab can trigger autoimmune reactions, such as pneumonitis, thyroiditis, or nephritis. These can be serious and require prompt medical attention.
- Tumor lysis syndrome: In patients with high tumor burden, rapid tumor cell death after Daratumumab treatment can lead to tumor lysis syndrome, a potentially life-threatening condition characterized by hyperkalemia, hyperuricemia, hyperphosphatemia, and hypocalcemia. Careful monitoring and supportive measures are essential.
Comparison of Safety Profiles
Daratumumab’s safety profile is generally comparable to other therapies used for hematologic malignancies, such as lenalidomide, bortezomib, and carfilzomib. However, specific adverse effects may differ depending on the drug and patient characteristics.
Adverse Events Categorized by Organ System
The following table summarizes the incidence and severity of adverse events associated with Daratumumab, categorized by organ system:
| Organ System | Adverse Event | Incidence | Severity |
|---|---|---|---|
| Hematologic | Neutropenia | Common | Mild to moderate |
| Thrombocytopenia | Common | Mild to moderate | |
| Anemia | Common | Mild to moderate | |
| Hemolytic anemia | Uncommon | Severe | |
| Gastrointestinal | Nausea | Common | Mild to moderate |
| Vomiting | Common | Mild to moderate | |
| Diarrhea | Common | Mild to moderate | |
| Constipation | Common | Mild to moderate | |
| Hepatic | Hepatotoxicity | Uncommon | Moderate to severe |
| Respiratory | Pneumonitis | Uncommon | Severe |
| Renal | Nephritis | Uncommon | Severe |
| Endocrine | Hypothyroidism | Uncommon | Mild to moderate |
| Immune | Infections | Common | Mild to severe |
| Autoimmune reactions | Uncommon | Severe |
Daratumumab in Clinical Trials
Daratumumab has been extensively evaluated in numerous clinical trials across various hematologic malignancies, demonstrating its efficacy and safety as a treatment option. These trials have provided valuable insights into the drug’s mechanism of action, optimal dosing regimens, and its potential in combination therapies. Ongoing clinical trials are further exploring the use of Daratumumab in new indications and exploring its synergistic effects with other therapies.
Key Findings from Major Clinical Trials
Major clinical trials have investigated the efficacy and safety of Daratumumab in multiple myeloma, Waldenström macroglobulinemia, and other hematologic malignancies. These studies have established Daratumumab as a valuable treatment option, particularly in patients with relapsed or refractory disease.
- The CASTOR trial, a phase III trial in patients with relapsed or refractory multiple myeloma, demonstrated that Daratumumab in combination with lenalidomide and dexamethasone significantly improved progression-free survival (PFS) and overall survival (OS) compared to lenalidomide and dexamethasone alone. The median PFS was 14.6 months with Daratumumab versus 7.6 months with lenalidomide and dexamethasone, and the median OS was 29.3 months versus 20.9 months, respectively. This study established Daratumumab as a standard of care for patients with relapsed or refractory multiple myeloma.
- The POLLUX trial, another phase III trial in patients with relapsed or refractory multiple myeloma, compared Daratumumab in combination with pomalidomide and dexamethasone to pomalidomide and dexamethasone alone. The results showed that Daratumumab significantly improved PFS and OS, with a median PFS of 18.1 months versus 9.7 months and a median OS of 36.4 months versus 28.4 months, respectively. These findings further solidified Daratumumab’s role in the treatment of relapsed or refractory multiple myeloma.
- The ALCYONE trial investigated the efficacy of Daratumumab in combination with bortezomib, melphalan, and prednisone (VMP) in patients with newly diagnosed multiple myeloma. The study demonstrated that Daratumumab significantly improved PFS and OS compared to VMP alone. The median PFS was 31.1 months with Daratumumab versus 20.2 months with VMP, and the median OS was not yet reached at the time of analysis. This trial established Daratumumab as a valuable treatment option for patients with newly diagnosed multiple myeloma.
- The GRIFFIN trial evaluated the efficacy of Daratumumab in combination with lenalidomide and dexamethasone in patients with newly diagnosed multiple myeloma who were ineligible for autologous stem cell transplantation. The results showed that Daratumumab significantly improved PFS and OS compared to lenalidomide and dexamethasone alone. The median PFS was 33.6 months with Daratumumab versus 22.4 months with lenalidomide and dexamethasone, and the median OS was not yet reached at the time of analysis. This trial further demonstrated the benefit of Daratumumab in the treatment of newly diagnosed multiple myeloma.
Ongoing Clinical Trials
Ongoing clinical trials are investigating the use of Daratumumab in new indications and combination therapies. These trials are exploring the potential of Daratumumab in treating various hematologic malignancies, including:
- Acute Lymphoblastic Leukemia (ALL): Trials are investigating the efficacy of Daratumumab in combination with other therapies for the treatment of ALL, particularly in patients with relapsed or refractory disease. This approach aims to leverage Daratumumab’s ability to target CD38, which is expressed on leukemia cells.
- Chronic Lymphocytic Leukemia (CLL): Studies are exploring the use of Daratumumab in combination with other therapies for the treatment of CLL, particularly in patients with relapsed or refractory disease. This strategy aims to improve treatment outcomes and address the challenges of CLL treatment.
- Hodgkin Lymphoma: Clinical trials are investigating the potential of Daratumumab in combination with other therapies for the treatment of Hodgkin lymphoma, particularly in patients with relapsed or refractory disease. This approach aims to enhance treatment efficacy and improve patient outcomes.
- Non-Hodgkin Lymphoma (NHL): Trials are investigating the use of Daratumumab in combination with other therapies for the treatment of NHL, particularly in patients with relapsed or refractory disease. This strategy aims to improve treatment outcomes and address the challenges of NHL treatment.
Summary of Major Clinical Trials
| Trial Name | Objective | Study Design | Key Results |
|---|---|---|---|
| CASTOR | Evaluate the efficacy and safety of Daratumumab in combination with lenalidomide and dexamethasone in patients with relapsed or refractory multiple myeloma. | Phase III randomized controlled trial. | Daratumumab significantly improved PFS and OS compared to lenalidomide and dexamethasone alone. |
| POLLUX | Evaluate the efficacy and safety of Daratumumab in combination with pomalidomide and dexamethasone in patients with relapsed or refractory multiple myeloma. | Phase III randomized controlled trial. | Daratumumab significantly improved PFS and OS compared to pomalidomide and dexamethasone alone. |
| ALCYONE | Evaluate the efficacy and safety of Daratumumab in combination with VMP in patients with newly diagnosed multiple myeloma. | Phase III randomized controlled trial. | Daratumumab significantly improved PFS and OS compared to VMP alone. |
| GRIFFIN | Evaluate the efficacy and safety of Daratumumab in combination with lenalidomide and dexamethasone in patients with newly diagnosed multiple myeloma who were ineligible for autologous stem cell transplantation. | Phase III randomized controlled trial. | Daratumumab significantly improved PFS and OS compared to lenalidomide and dexamethasone alone. |
Future Directions for Daratumumab Research
Daratumumab, a monoclonal antibody targeting CD38, has revolutionized the treatment of multiple myeloma and other hematologic malignancies. Its success has spurred ongoing research to further expand its therapeutic potential and address existing limitations.
Combination Therapies
The combination of Daratumumab with other therapies has proven highly effective in treating multiple myeloma. Future research aims to explore novel combinations with emerging agents, such as CAR T-cell therapy, bispecific antibodies, and immunomodulatory drugs. These combinations hold the promise of synergistic effects, leading to improved response rates, deeper remissions, and potentially even cures.
- CAR T-cell therapy: Combining Daratumumab with CAR T-cell therapy could enhance the efficacy of CAR T-cells by targeting CD38-expressing myeloma cells and reducing the tumor burden. This approach could overcome limitations of CAR T-cell therapy, such as resistance to therapy and tumor heterogeneity.
- Bispecific antibodies: Bispecific antibodies targeting both CD38 and other myeloma-associated antigens could simultaneously engage multiple pathways, leading to enhanced tumor cell killing and immune activation.
- Immunomodulatory drugs: Combining Daratumumab with immunomodulatory drugs, such as lenalidomide and pomalidomide, could enhance the anti-tumor immune response by promoting the activation and proliferation of immune cells.
New Indications
Daratumumab’s efficacy in multiple myeloma has opened doors to explore its potential in other hematologic malignancies and solid tumors. Ongoing clinical trials are investigating its use in various cancers, including:
- Acute myeloid leukemia (AML): Daratumumab has shown promising results in preclinical studies for AML, suggesting its potential to target CD38-expressing AML cells.
- Chronic lymphocytic leukemia (CLL): Clinical trials are evaluating Daratumumab’s effectiveness in treating CLL, a type of leukemia characterized by the accumulation of mature B-cells.
- Non-Hodgkin lymphoma (NHL): Daratumumab’s potential in NHL is being investigated in clinical trials, targeting CD38-expressing lymphoma cells.
- Solid tumors: Preclinical studies suggest Daratumumab’s potential in solid tumors, such as breast cancer, lung cancer, and melanoma, by targeting CD38-expressing tumor cells.
Novel Drug Delivery Systems
Current research focuses on developing novel drug delivery systems for Daratumumab to enhance its efficacy and reduce its side effects. These systems aim to improve drug targeting, increase bioavailability, and prolong drug exposure.
- Nanoparticle-based delivery: Encapsulating Daratumumab in nanoparticles could enhance its delivery to tumor cells, improve its pharmacokinetic profile, and potentially reduce its off-target effects.
- Antibody-drug conjugates (ADCs): Combining Daratumumab with cytotoxic drugs through ADC technology could target tumor cells more effectively, reducing the risk of systemic toxicity.
Optimizing Efficacy and Safety
Ongoing research aims to optimize Daratumumab’s efficacy and safety by investigating different dosing regimens, combination therapies, and personalized approaches.
- Dosing regimens: Studies are exploring different dosing schedules and frequencies to maximize Daratumumab’s effectiveness and minimize its side effects.
- Biomarkers: Researchers are investigating biomarkers that can predict patient response to Daratumumab, allowing for personalized treatment strategies.
- Safety monitoring: Ongoing research focuses on developing strategies to monitor and manage Daratumumab-related adverse events, such as infusion reactions and immune-mediated adverse events.
Personalized Medicine Approaches
The use of Daratumumab in personalized medicine approaches holds great promise for tailoring treatment to individual patient characteristics and tumor biology.
- Genomic profiling: Identifying genetic mutations and biomarkers associated with Daratumumab sensitivity could allow for more precise patient selection and treatment optimization.
- Immunophenotyping: Characterizing the immune profile of patients could guide the use of Daratumumab in combination with other therapies to enhance the immune response against cancer cells.
- Liquid biopsy: Monitoring tumor evolution through liquid biopsy could enable early detection of resistance to Daratumumab and allow for timely adjustments to treatment strategies.
Economic Considerations of Daratumumab
Daratumumab, a monoclonal antibody targeting CD38, has shown significant efficacy in treating hematologic malignancies, particularly multiple myeloma. However, its high cost raises important economic considerations. This section delves into the cost-effectiveness of Daratumumab compared to other therapies, its impact on healthcare systems and patient access, and strategies for optimizing its use.
Cost-Effectiveness of Daratumumab
The cost-effectiveness of Daratumumab is a crucial aspect of its clinical application. Studies have evaluated its cost-effectiveness compared to other treatment options, including standard therapies and other novel agents.
Cost-effectiveness analysis typically involves comparing the cost of a treatment with its effectiveness in achieving clinical outcomes, such as survival or quality of life.
Several factors contribute to the cost-effectiveness of Daratumumab, including:
- Improved survival rates: Daratumumab has demonstrated improved overall survival and progression-free survival in various clinical trials, leading to potential cost savings in the long term.
- Reduced treatment burden: Daratumumab’s convenient intravenous administration and longer dosing intervals can potentially reduce healthcare utilization and costs associated with frequent hospital visits and treatment administration.
- Potential for cost-offsetting: The improved outcomes with Daratumumab may lead to reduced costs associated with complications, hospitalizations, and supportive care, potentially offsetting the high cost of the drug.
Impact on Healthcare Systems and Patient Access
The high cost of Daratumumab poses challenges for healthcare systems and patient access to treatment.
- Budgetary constraints: The high price of Daratumumab can strain healthcare budgets, particularly in resource-limited settings.
- Limited access to treatment: High drug costs can limit patient access to Daratumumab, potentially exacerbating health disparities and hindering the availability of effective treatment options.
- Impact on insurance coverage: The cost of Daratumumab can influence insurance coverage policies and create challenges for patients in obtaining prior authorization and coverage for treatment.
Strategies to Optimize Daratumumab Use
To optimize the use of Daratumumab and minimize its overall cost, several strategies can be implemented:
- Early identification of suitable candidates: Identifying patients who are most likely to benefit from Daratumumab can optimize its use and avoid unnecessary expenses.
- Negotiating drug prices: Healthcare systems and payers can negotiate with pharmaceutical companies to secure lower drug prices for Daratumumab, improving affordability and access.
- Developing cost-effective treatment regimens: Optimizing treatment regimens, including dose adjustments and combination therapies, can potentially reduce the overall cost of Daratumumab therapy.
- Utilizing value-based pricing models: Value-based pricing models that link drug prices to clinical outcomes can incentivize the development of cost-effective therapies and ensure that patients receive the most value for their healthcare expenditures.
Patient Perspectives on Daratumumab
Living with multiple myeloma is a challenging journey, and treatment options can significantly impact a patient’s quality of life. Daratumumab, a monoclonal antibody therapy, has emerged as a promising treatment for this disease, offering potential benefits in terms of survival and disease control. However, understanding the patient experience with Daratumumab is crucial for informed decision-making. This section explores patient testimonials, challenges, and benefits associated with Daratumumab treatment, shedding light on the impact of this therapy from a personal perspective.
Patient Experiences with Daratumumab
Patients receiving Daratumumab treatment often share their experiences and insights, providing valuable information for others navigating this journey. Testimonials highlight the diverse impact of Daratumumab on quality of life, treatment burden, and overall outcomes.
- Improved Quality of Life: Many patients report an improved quality of life while on Daratumumab treatment. This includes increased energy levels, reduced pain, and better overall well-being. Some patients experience a reduction in the frequency and severity of myeloma-related symptoms, allowing them to participate more actively in their daily lives.
- Treatment Burden: Daratumumab is typically administered intravenously, which can involve frequent clinic visits and potential side effects. Some patients may experience infusion reactions, fatigue, or other adverse events, which can impact their daily routines. However, many patients report that the benefits of Daratumumab outweigh the potential drawbacks.
- Overall Outcomes: Daratumumab has demonstrated promising results in clinical trials, leading to improved survival rates and disease control for many patients. Patients often express hope and optimism about the potential for Daratumumab to help them manage their myeloma and live longer, healthier lives.
Challenges of Receiving Daratumumab Treatment
While Daratumumab offers potential benefits, patients may face various challenges during treatment. These challenges can be related to the treatment itself, its side effects, or the impact on their daily lives.
- Infusion Reactions: Infusion reactions are a common side effect of Daratumumab, ranging from mild to severe. These reactions can include fever, chills, nausea, and shortness of breath. Patients may need to be closely monitored during infusions and receive pre-medication to minimize the risk of reactions.
- Fatigue: Fatigue is another common side effect of Daratumumab, which can impact a patient’s energy levels and ability to participate in daily activities. Patients may need to adjust their schedules and seek support from family and friends to manage fatigue.
- Financial Burden: Daratumumab is an expensive treatment, which can pose a financial burden for some patients. Insurance coverage and access to financial assistance programs can vary, making it essential for patients to explore their options and seek support if needed.
Benefits of Receiving Daratumumab Treatment
Daratumumab offers numerous benefits for patients with multiple myeloma, potentially improving their quality of life, extending their survival, and providing hope for the future.
- Improved Survival: Daratumumab has been shown to improve survival rates for patients with multiple myeloma, particularly when used in combination with other therapies. This extended survival allows patients to spend more time with their loved ones and enjoy life to the fullest.
- Disease Control: Daratumumab can effectively control the progression of multiple myeloma, reducing the risk of disease-related complications and improving overall health. This control allows patients to live with less pain and discomfort, enabling them to engage in activities they enjoy.
- Improved Quality of Life: Daratumumab can improve the quality of life for patients with multiple myeloma by reducing symptoms, increasing energy levels, and improving overall well-being. This allows patients to live more fulfilling and meaningful lives.
Patient-Reported Outcomes Associated with Daratumumab Therapy
| Outcome | Description |
|---|---|
| Quality of Life | Patient-reported measures of overall well-being, including physical, emotional, and social aspects. |
| Fatigue | Patient-reported levels of tiredness, exhaustion, and lack of energy. |
| Pain | Patient-reported levels of pain intensity, frequency, and impact on daily activities. |
| Treatment Satisfaction | Patient-reported satisfaction with the overall treatment experience, including effectiveness, side effects, and convenience. |
| Emotional Well-being | Patient-reported measures of anxiety, depression, and overall mood. |
Daratumumab has emerged as a valuable tool in the armamentarium against hematologic malignancies. Its targeted approach, coupled with its efficacy and safety profile, has made it a cornerstone of modern treatment regimens. As research continues, we can anticipate further advancements in its use, including novel combinations and personalized treatment strategies, ultimately leading to improved patient outcomes and a better understanding of this promising therapy.
Daratumumab is a monoclonal antibody used to treat multiple myeloma, a type of blood cancer. It works by targeting CD38, a protein found on the surface of myeloma cells. While daratumumab is a powerful tool in the fight against myeloma, it’s important to consider other medications like sandostatin , which is used to treat acromegaly, a condition caused by excessive growth hormone production.
This underscores the need for a multi-faceted approach to cancer treatment, incorporating different therapies to address the unique needs of each patient.